Abstract
Background
For favorable- and intermediate-risk AML patients who lack of an available HLA-matched donor, the post-remission treatment is controversial. Autologous stem cell transplantation (auto-SCT) and haplo-identical stem cell transplantation (haplo-SCT) are widely used alternative options, both of which revealed promising data according to recent studies. However, few data have been published on the direct comparison between these two transplant types.
Patients and Methods
This is a retrospective study based on the transplantation database in our center. The inclusion criteria were: a) patients who were definitely diagnosed as de novo AML without extramedullary disease; b) who achieving complete remission (CR) after induction chemotherapy of no more than 2 cycles; c) who had results of cytogenetic and molecular genetic detections suggesting a favorable- or intermediate-risk according to the NCCN guideline of AML; and d) who received auto-SCT or haplo-SCT in CR1 before January 2014. Myeloablative conditioning applied in this cohort was Bu/Cy-based regimen (with ATG for haplo-SCT recipients), and a minority of auto-SCT patients received a nonmyeloablative conditioning of BEAM regimen.
Results
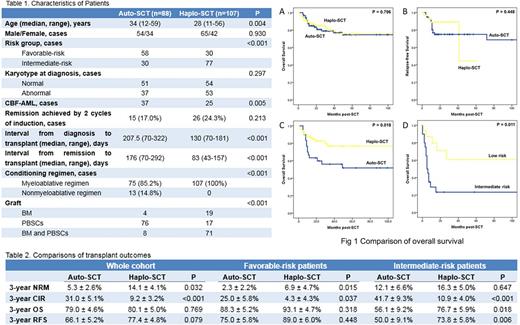
Totally 196 cases were recruited in this study, in which 88 cases received auto-SCT and 107 cases received haplo-SCT (Table 1). There are statistical differences of age, risk stratification, ratio of CBF-AML, induction cycles, interval from diagnose or CR1 to transplant, conditioning and graft type between the two groups. After SCT, neutrophil recovery was earlier in auto-SCT group with a median time of 11 days comparing to 12 days in haplo-SCT group (P = 0.004). Survival, relapse and NRM were shown in Table 2. For the whole cohort and favorable-risk patients, impact of high relapse incidence in auto-SCT group was compensated by low NRM, which resulted in a comparable survival comparing to haplo-SCT group (Fig 1A and 1B). However, for intermediate-risk patients, NRM was similar between the two groups and haplo-SCT exhibited superior survival (Fig 1C).
Thirty-six cases relapsed after SCT with a median time of 9 months post-SCT, in which 28 cases received auto-SCT and 8 cases received haplo-SCT. Estimated OS at 3 years for relapsed patients was 38.7 ± 9.0%, without notable difference between auto-SCT and haplo-SCT (37.9 ± 9.9% versus 40.0 ± 20.3%, P = 0.823). Instead, patients with intermediate-risk showed markedly inferior 3-year OS comparing to the ones with favorable-risk (23.3 ± 9.8% versus 60.8 ± 14.3%, P=0.011) (Figure 1D). For intermediate-risk patients, only 5 out of 20 (25.0%) cases who relapsed post-SCT could achieved constant remission again. Contrarily for favorable-risk patients, 11 out of 16 (68.8%) cases achieved remission again (P = 0.017), and 10 cases in whom remained survival at the end of follow-up.
In the multivariate analyses, MRD measured by FCM and gene mutation status before transplant were independent predict factor for both OS and RFS. Interestingly, intermediate-risk independently led to an inferior OS rather than RFS, probably due to the poorer survival of post-SCT relapse for intermediate-risk cases. Transplant type had no independent impact on OS and RFS, but haplo-SCT could result in higher risk of NRM and lower risk of relapse. In addition, myeloablative conditioning and longer interval from diagnosis to transplant will increase the risk of NRM, while positive gene mutation before transplant will increase the risk of relapse post-SCT.
Conclusion
Both auto-SCT and haplo-SCT were acceptable option as post-remission treatment for favorable- and intermediate-risk AML patients. For intermediate-risk cases, haplo-SCT revealed better survival but the relapse after SCT still led to a poor outcome. Clearance of MRD before SCT could improve the prognosis after transplantation.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal